Jane Synnergren
School of Bioscience
The advancement in stem cell research over the last decade has now made it possible to generate large quantities of human specialized cells for in vitro applications. Specifically, in the drug discovery and development process this has important implications. The project involves studies of the genetic and molecular basis of hypertrophy and aims to develop new knowledge that can contribute to the development of novel therapies and treatments that can reduce cardiovascular morbidity and mortality.
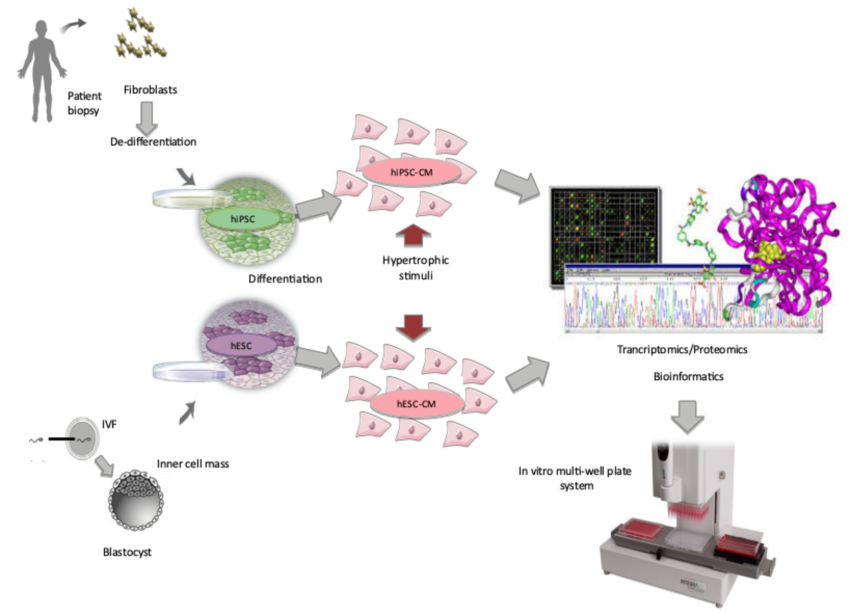
The generation of human cardiomyocytes from pluripotent stem cells allows the establishment of clinically relevant in vitro systems to test unwanted side-effects of novel drug candidates.
Previously, the access to human cardiomyocytes has been limited, and pharmaceutical industries have had to rely on non-human models for pre-clinical testing. These models have limited human relevance, which contribute to costly late stage attrition in drug development projects.
This project addresses the need for new more advanced in vitro models to test adverse responses in human cardiomyocytes with a specific focus on cardiac hypertrophy.
The project involves studies of the genetic and molecular basis of hypertrophy and aims to develop new knowledge that can contribute to the development of novel therapies and treatments that can reduce cardiovascular morbidity and mortality. To this end, we are generating cardiomyocytes from human pluripotent stem cells for use in assay development.
By exposing these cardiomyocytes to substances that are known to induce cardiac hypertrophy e.g. Endothelin-1 we are inducing a disease phenotype in vitro that mimic cardiac hypertrophy in vivo. This model is used to characterize the hypertrophic response on a mechanistic level using large-scale omics technologies such as transcriptomics and proteomics.
These technologies generate large amounts of data, which is used to identify critical biomarkers that may constitute novel targets to assess cardiac hypertrophy. Our optimal goal is then to use this experimental system in combination with the biomarker(s) identified to develop, optimize, and implement a complete assay for human cardiac hypertrophy in a multi-well format that can be used in compound screens to assess if they induce any deleterious hypertrophic effects in the cells.